In the past 20 years, deep brain stimulation (DBS) has been used for over 100,000 patients with Parkinson’s disease. The success of this procedure has led investigators to try DBS for other neurologic conditions, such as Alzheimer’s disease (AD).
In 2016, Lozano et al reported on one of the largest trials for DBS in AD, the “ADvance” trial, in which they targeted the fornix, a bundle of nerve fibers in the center of the brain that is the major output tract of the hippocampus.
This was a well-run, double-blind, randomized study. One of the nice aspects about brain stimulation trials is the ease of performing a sham stimulation arm. That is, treatment can be randomly turned either “on” and “off” for a period of time, allowing a subset of participants to serve as controls (stimulation turned “off”) for a period of time before they actually do get the stimulation (stimulation turned “on”) in case it is actually helpful.
In terms of the trial results, one of the patients (out of 42) had an implant infection. Overall, the trial did not show a significant benefit mitigating the decline in ADAS-13 or CDR-SB scores (measures of cognitive function):
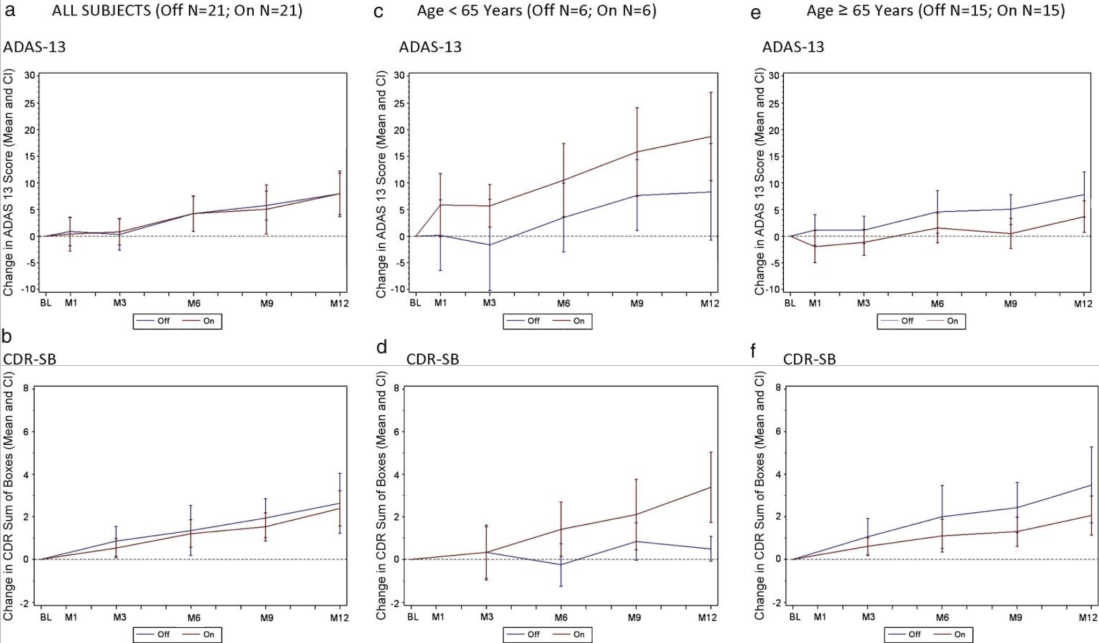
While this trial did not show efficacy at their sample sizes, personally I expect that DBS for early AD could work to at least alleviate symptoms, if the right circuits were targeted at the right time.
My reasoning here is that we know that a few other cognitive strategies can help slow the course of AD, including processing speed training and acetylcholinesterase inhibitors.
There are at least 4 active DBS trials for AD on clinicaltrials.gov:
- One trial at Hospital San Carlos, Madrid, studying the effect of DBS in the fornix compared to the nucleus basalis.
- One trial at Beijing Tiantan Hospital, studying the PINS stimulation device (unclear brain region).
- One trial at Chinese PLA Hospital, Beijing, studying DBS of the fornix.
- One trial at UCLA Longevity Center, studying a Low Intensity Focused Ultrasound Pulsation procedure to either activate or inhibit hippocampal neurons as a method to treat AD.
It will be interesting to monitor this growing field in the coming years.